Weekly Roundup is a McNair Center series compiling and summarizing the week’s most important Entrepreneurship and Innovation news.
Here is what you need to know about entrepreneurship this week:
How to Make Texas More Startup-Friendly
Iris Huang, Research Assistant, McNair Center for Entrepreneurship and Innovation
McNair Center’s Huang interviews Blake Commagere, entrepreneur, angel investor and startup mentor in the San Francisco Bay Area on how to improve an entrepreneurial ecosystem. Commagere graduated from Rice University in 2003 with a degree in Computer Science. Upon graduation, Commagere moved to Austin to begin his career as an entrepreneur and soon decided to move to Silicon Valley. Commagere has raised over $12 million in VC, started seven companies and sold five.
Commagere describes the pull of talent toward San Francisco as “a virtuous cycle,” where “former successful startup founders become the next generation angel investors and venture capitalists, who fund and help more startups succeed.” Silicon Valley’s concentrated network of VC firms and tech startups provide struggling entrepreneurs with a vast pool of mentorship opportunities, funding resources and talent. Budding startups heavily rely on local tech networks for early-stage support and advice. In order to develop its entrepreneurial ecosystem, Texas cities need to focus on building its tech space.
Additionally, the state’s cities must expand their VC presence. Otherwise, there will always be too many startups fighting for too little capital (as if this isn’t a problem already), and startups will continue to move to cities like San Francisco. Startups depend on local VC firms because many firms refrain from investing in companies outside their primary city. When firms do invest in outside companies, the qualification bar is set much higher.
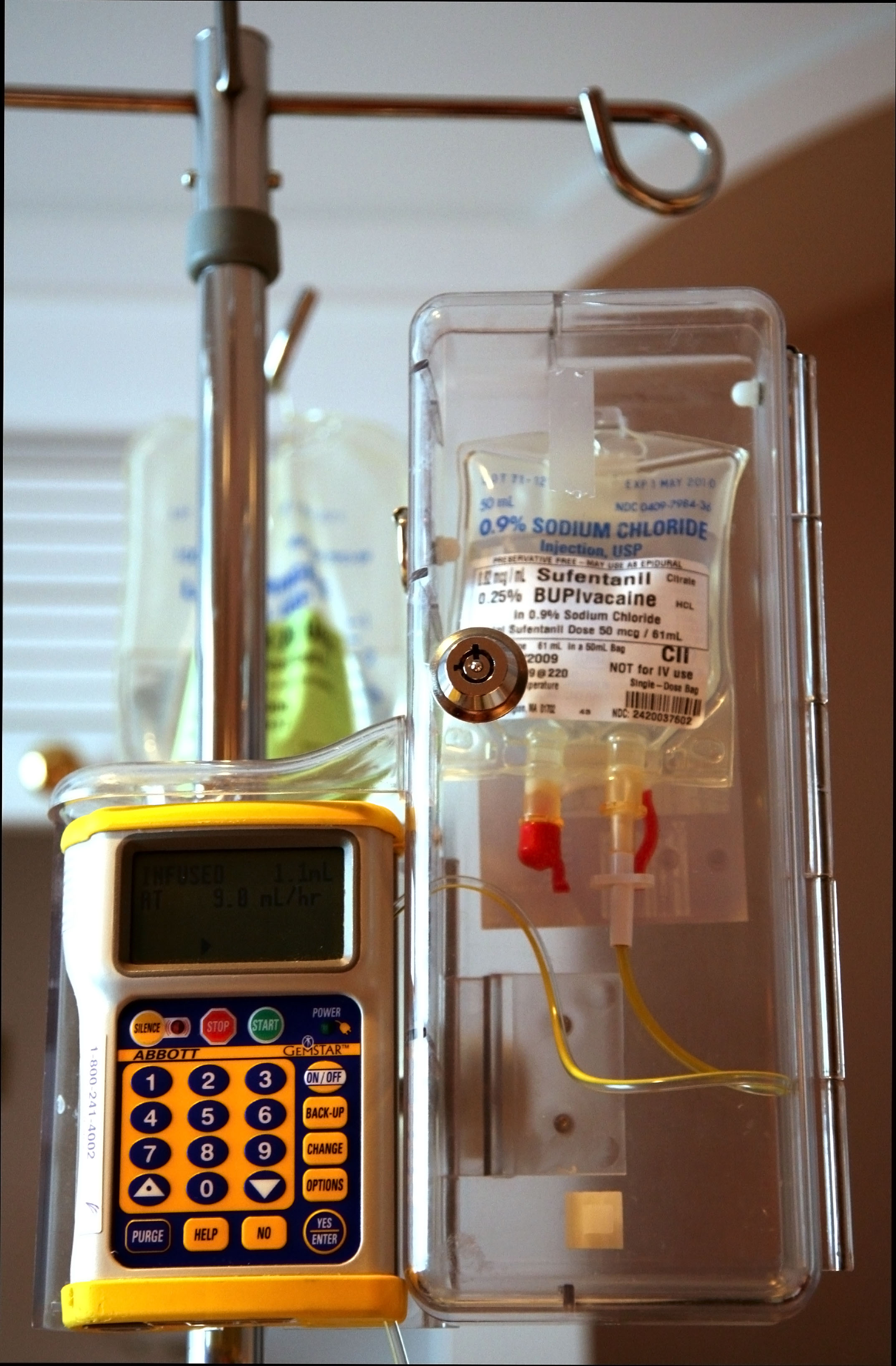
Medical Device Startups and the FDA
Iris Huang, Research Assistant, McNair Center for Entrepreneurship and Innovation
McNair Center’s Huang takes a look at the FDA approval process for medical devices. The medical device industry is a $140 billion market. For many companies in the industry, obtaining FDA approval is a long and costly path. For some, it’s a barrier. Of the 6,500 companies in medtech, 80 percent are composed of fewer than 50 employees.
A Stanford University survey of over 200 medtech companies found that the average cost for a low-to-moderate-risk 510(k) product to obtain FDA clearance was $31 million. The same survey found that it took these products 31 months from initial communications with the FDA to obtain clearance. For startups, these costs pose significant barriers to entry. Huang aptly summarizes this dilemma: “as the cost of getting to market approaches the average exit value, the medtech funding equation looks less attractive to venture capitalists.”
The FDA approval process acts as an essential screening point in the medtech industry. However, Huang recommends that policymakers consider possible ways to alleviate the significant burdens placed on the businesses involved in the development of these critical technologies.
First Data Joins Silicon Valley Bank In Fintech Accelerator
Tom Groenfeldt, Contributor, Forbes
Silicon Valley Bank (SVB) recently announced a collaboration with First Data, a global payments technology solutions company, on Commerce.Innovated, its fintech accelerator. Commerce.Innovated, founded in 2014, is a four-month long virtual accelerator for startups in the financial services and technologies sector. The accelerator, unlike most early stage accelerators, focuses on startups that have already secured or are in the process of securing seed or Series A funding.
According to SVB’s Reetika Grewal, the accelerator looks for firms with “five to 10 people with an idea they are committed to.” In this stage, startups usually require help with the “operational,” rather than conceptual, front of development. Commerce.Innovated helps fintech firms bring their solutions to market. Since these startups already possess strong leadership with a clear vision for their product, a virtual platform makes sense.
A $150 Million Fund, The Engine, Will Back Startups Others Find ‘Too Hard’
Lora Kolodny, Contributor, TechCrunch
The Engine is a venture fund and accelerator for “advanced technology startups.” The new fund recently closed its debut round at $150 million. Startups in The Engine’s portfolio gain access to one of MIT’s unique resources, The Engine Room, a laboratory for small startups to develop and test their technologies. In addition to to The Engine Room, startups also receive access to laboratory equipment and technologies from organizations in the greater Boston area.
Despite its close affiliation to MIT, The Engine invests “in teams and technologies that hail from a variety of industry and academic backgrounds, not just from the MIT ecosystem.” The Engine supports companies involved in the development of “hard-tech” – so basically anything “from advanced materials and manufacturing technologies to medical devices, robotics, artificial intelligence, nuclear energy, fusion and more.”
Hard-tech startups typically face higher costs, more risk and a longer development period than most B2B or consumer-focused software. These startups often find it difficult to find VCs willing to invest in their innovative, but risky technologies. The Engine, according to the fund’s CEO Katie Rae, is dedicated to lowering the costs of development and testing “hard-tech” and encouraging more entrepreneurs to go into the field.
Tax Reform Must Help Small Businesses, Too
Laurie Sprouse, Reporter, The Wall Street Journal
Laurie Sprouse, a small business owner from Dallas, covers tax reform and small businesses for The Wall Street Journal. As Sprouse points out, small businesses have added two thirds of new jobs to the U.S. economy in recent years. Still, analysts and policymakers continually propose tax overhauls that largely ignore the plight of small firms. Instead, politicians and reporters alike focus on alleviating financial burdens for larger corporations and providing helpful, but insufficient, tax credits for small businesses. According to Sprouse, “Only a plan that benefits businesses of all sizes equally will create the broad economic growth President Trump and Congress seek.”
Stripe Acquires Indie Hackers in Bid to Strengthen Relationship with Entrepreneurs
Ken Yeung, Contributor, VentureBeat
Founded in 2010, tech company Stripe delivers application programming interfaces (APIs) that support electronic payments for consumers and businesses. Recently, the firm announced plans to acquire Indie Hackers, a startup dedicated to creating an internet community for entrepreneurs to share their success stories and lessons. While the financial terms of the deal remain unclear, it seems that site will operate as an independent subsidiary of Stripe.
Indie Hackers founder, Courtland Allen, describes his site as a “community where successful founders could share their valuable stories and insights, and where aspiring entrepreneurs could go for inspiration and advice.” Meanwhile Stripe executives view the deal as an opportunity to grow “the GDP of the internet” by increasing the “overall number” of successful businesses.
In an interview with VentureBeat, a Stripe spokesperson revealed that the company wants to support Indie Hackers’ mission by taking on some of the budding site’s financial burden. In just under a year, the site already runs a monthly profit of $6,000. Going forward, Allen hopes to see Indie Hackers take on a similar role as Y Combinator’s Hacker News.
The Weekly Roundup will return in June.